(Brand Name: Faverin, Luvox)
The following information is a guide only and you must discuss your medication needs with a trained health professional.
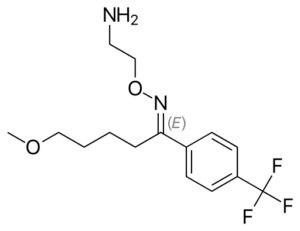
Fluvoxamine was the first SSRI approved for clinical use here in the United Kingdom, and was developed by Kali-Duphar in Belgium and introduced as Floxyfral in Switzerland and Solvay in West Germany in 1983. It was approved by the FDA on 5 Dec, 1994 and introduced as Luvox in the US.
Key Facts:
- Check with your doctor before starting to take Fluvoxamine if you are trying to become pregnant, or plan to discontinue it if you’re already pregnant or you are breastfeeding
- Fluvoxamine can cause withdrawal symptoms so don’t stop taking it without talking to your doctor
- In the United States, Fluvoxamine carries a warning stating it may increase suicidal thinking and behaviour in those under age 24
Medication Information
The following information is a guide only, a doctor may want to try dosages outside these recommended guides.
When: In the evening before bedtime.
Child dose: Consult a doctor.
Adult dose: The usual initial dose for adults is 50mg daily and is increased gradually if necessary up to a maximum of 300 mg daily.
Older people: Consult a doctor.
Half-Life: Around 12–13 hours for a single dose, increasing up to 22 hours for regular use.Pregnancy and Breast Feeding: It’s important for you and your baby that you stay well during your pregnancy. If you become pregnant while taking Fluvoxamine speak to your doctor, it’s important not to stop taking your medicine unless your doctor tells you to. Ask your doctor to discuss the potential benefits against potential risks of staying on medication whilst pregnant or breastfeeding so that you can make an informed choice about what is right for you and your baby.
The NICE guidelines, published in 2005, reported that Fluvoxamine has a UK marketing authorisation for treating OCD in children aged 8 years and older.
What to read next: